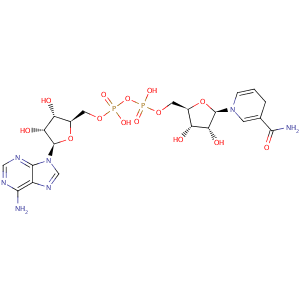
NADH
NADH synonyms
Graphical representations:
View large 3D structure
Molecular Formula: C21H29N7O14P2
Natural Isotopic Abundance Mass: 665.4409820000
Mono-Isotopic Molecular Masses:
- C12N14: 665.124771697
- C13N14: 686.19522329
- C12N15: 672.104015949
- C13N15: 693.1744675429
InChI string: InChI=1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1
IUPAC: [(2R,3R,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[[(2R,3R,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-phosphinic acid
PubChem Compound (CID): 439153KEGG Compound ID: C00004
ChEBI: 16908
PDB Compound ID: NAI
Reference data were obtained primarily from the PubChem database.
Three dimensional molecular rendering uses Jmol.
InChI string and atom numbering calculated using ALATIS (Hesam Dashti, William M. Westler, John L. Markley, Hamid R. Eghbalnia, "Unique identifiers for small molecules enable rigorous labeling of their atoms", Scientific Data 4, Article number: 170073 (2017), doi:10.1038/sdata.2017.73, https://www.nature.com/articles/sdata201773)